Atomic Layer Deposition (ALD)
The ALD is based on the chemical vapor deposition (CVD) process, where the chemical reaction is separated into two half-reactions. As shown in the figure 5, the process starts by a pulse of metal-organic precursor gas in to the deposition chamber. Under certain conditions, the gas reacts with the surface species of the substrate in a self-limiting reaction that is terminated when the surface runs out of reactants. The excess gas is purged in the second step with a neutral gas such as N2 or Ar depending on the process requirements. The second reactant is introduced into the chamber in the third step, again reacting with the surface species. The excess of reactant and products are purged in the fourth step, concluding one cycle. In an ideal ALD process one atomic layer of material is deposited in each cycle and the number of cycles determines the thickness of the deposited film. ALD deposited films are highly conformal and can be used for coating and encapsulation of complex geometries.
ALD is especially suited for depositing thin films of ceramic materials such as oxides and nitrides. So far, we have successfully deposited Al2O3, SiO2, Ta2O5, HfO2 and Pt and their multilayers in our group. We have the possibility to extend this to other oxides and nitrides.
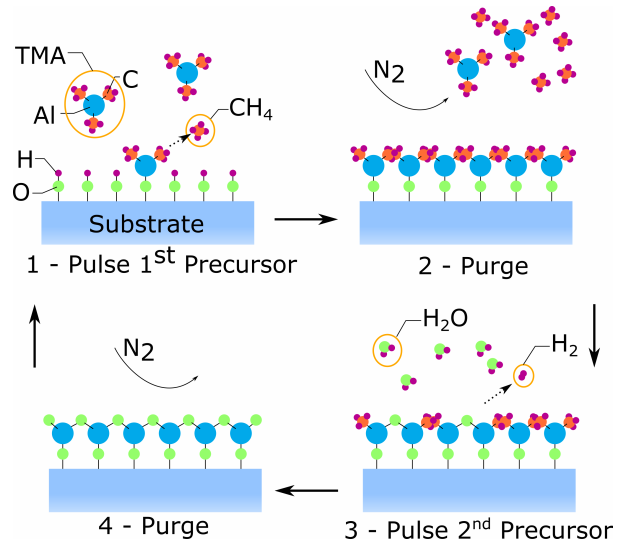
An example ALD cycle for the deposition of Al2O3.
